In the realm of software development, the focus is often on creating efficient, reliable, and innovative code that powers everything from our daily apps to critical medical devices. However, the potential for catastrophic failure looms large when errors in code can lead to deadly consequences. This post delves into one of the most tragic instances in the history of computing: the code that killed six people, infamously known as the case of Ray Cox 86. This educational piece will explore the history, implications, and lessons learned from this fatal flaw, shedding light on the significance of robust software engineering practices.
In the mid-1980s, a series of tragic events unfolded that highlighted the deadly consequences of software errors. The Therac-25, a radiation therapy machine used for treating cancer patients, was involved in several fatal accidents due to a software bug. Between 1985 and 1987, six patients received massive overdoses of radiation, leading to severe injuries and, in some cases, death.
The Machine and Its History
The Therac-25 was developed by Atomic Energy of Canada Limited (AECL) and was an advanced version of previous models, the Therac-6 and Therac-20. These machines combined a linear accelerator with a computer system to deliver precise doses of radiation. The Therac-25 was intended to be safer and more efficient, relying heavily on software controls rather than hardware interlocks, which were present in earlier models.
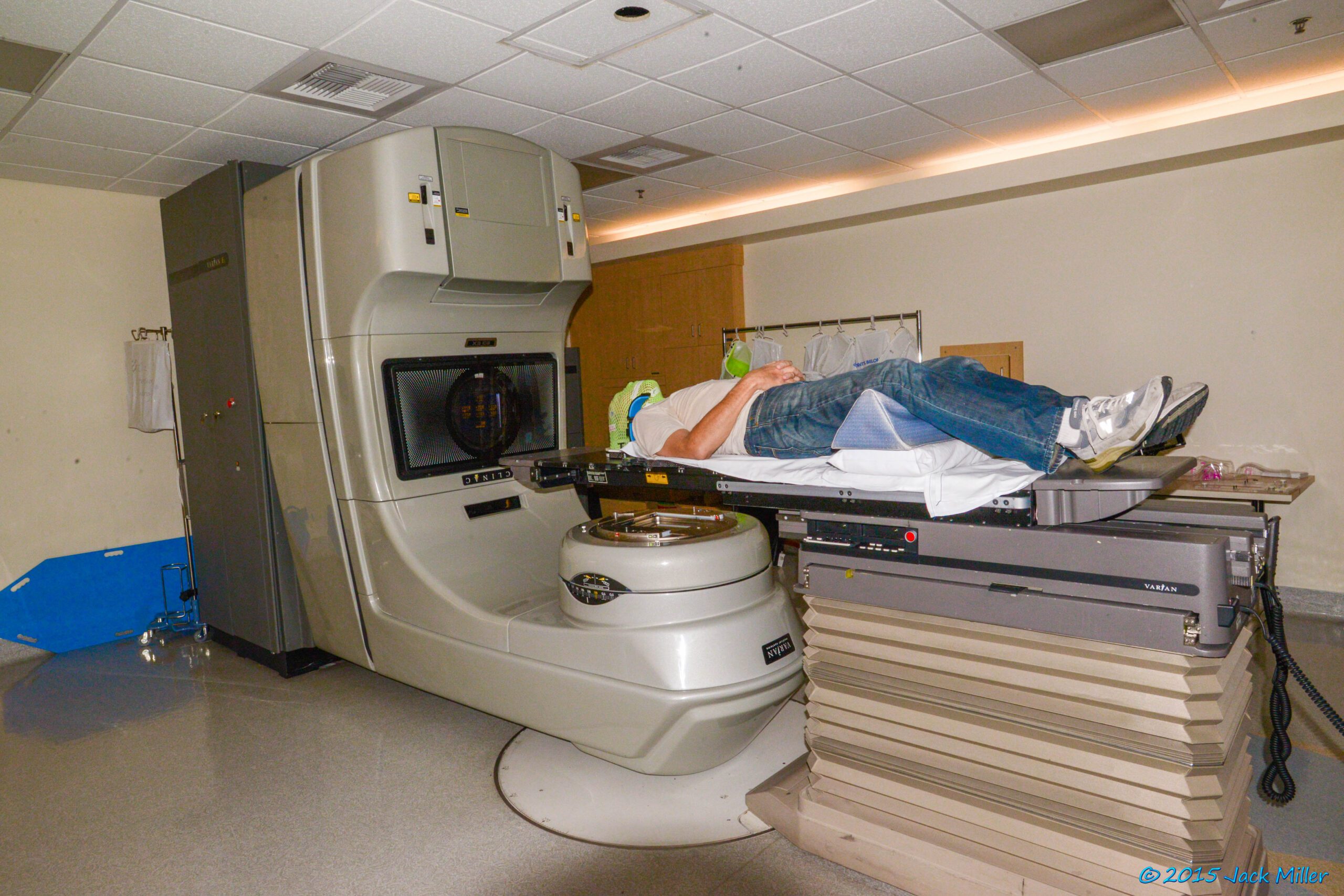
The Fatal Code
The software error in the Therac-25 was subtle yet catastrophic. The machine’s control software had a race condition, a type of bug that occurs when the timing of events affects the program’s behavior. Specifically, if the operator entered commands too quickly, the software could misinterpret the state of the machine, leading to the beam being activated at a much higher power than intended.
One notable incident involved Ray Cox, referred to as Ray Cox 86, who was a victim of the Therac-25’s malfunction. He received a massive overdose of radiation, resulting in severe burns and ultimately his death. The subsequent investigation revealed that the machine had delivered a radiation dose 100 times higher than prescribed.
The Impact and Lessons Learned
The Therac-25 incidents profoundly impacted the field of software engineering and medical device safety. Several critical lessons emerged from these tragedies:
1. Importance of Robust Testing
The Therac-25’s software had not undergone rigorous testing. It was assumed that the new software would work seamlessly with the existing hardware, an assumption that proved fatal. This highlights the need for comprehensive testing, particularly in safety-critical systems.
2. The Role of Redundancy and Hardware Interlocks
The removal of hardware interlocks in favor of software controls was a significant factor in the Therac-25 incidents. Hardware interlocks provide a physical safeguard against software errors. In safety-critical systems, relying solely on software without adequate hardware backups can be disastrous.
3. Transparent Reporting and Incident Tracking
The initial accidents involving the Therac-25 were not adequately reported or tracked, delaying the identification of the underlying issue. Transparent reporting mechanisms and incident tracking are essential for quickly identifying and addressing safety issues.
4. Formal Methods and Verification
The incidents underscored the need for formal methods and rigorous verification processes in software development. Formal methods involve mathematically proving that a system’s design and implementation meet specified requirements, reducing the likelihood of errors.
Historical Context and Industry Response
The Therac-25 incidents occurred at a time when software was becoming increasingly integral to medical devices and other critical systems. The tragedies prompted significant changes in how software for such systems is developed, tested, and regulated.
Regulatory Changes
In the aftermath of the Therac-25 incidents, regulatory bodies like the U.S. Food and Drug Administration (FDA) implemented stricter guidelines for the development and testing of medical device software. These guidelines emphasize the importance of risk management, thorough testing, and comprehensive documentation.
Industry Standards
The incidents also led to the development of industry standards for software safety. For example, the International Electrotechnical Commission (IEC) published the IEC 62304 standard, which outlines requirements for the lifecycle processes of medical device software. This standard provides a framework for ensuring that software is developed and maintained to the highest safety standards.
The Ethical Dimension
The Therac-25 case also raises important ethical questions about the responsibility of software developers and engineers. When developing software for safety-critical systems, engineers must consider the potential consequences of their work and prioritize safety above all else.
Ethical Considerations
- Accountability: Software developers and companies must be held accountable for the safety and reliability of their products. This includes transparent reporting of incidents and proactive measures to prevent future occurrences.
- Continual Learning: The field of software engineering must continually evolve, learning from past mistakes to improve future practices. The Therac-25 incidents serve as a stark reminder of the importance of continual learning and improvement.
- User Training and Awareness: Operators of safety-critical systems must be adequately trained and aware of the potential risks. In the case of the Therac-25, better training and awareness could have helped prevent some of the incidents.
Looking Forward: Ensuring Safety in Software Development
The lessons learned from the Therac-25 incidents continue to shape the field of software engineering. Ensuring the safety and reliability of software, particularly in safety-critical systems, requires a multi-faceted approach:
- Adopting Best Practices: Developers must adopt best practices for software development, including rigorous testing, formal methods, and risk management.
- Continuous Improvement: The field must continually evolve, learning from past mistakes and incorporating new techniques and technologies to improve safety.
- Collaboration: Collaboration between developers, regulatory bodies, and industry stakeholders is essential to ensure that safety standards are met and maintained.